Abstract
Background: The oncogenic drivers and progression factors in multiple myeloma (MM) are heterogeneous and difficult to target therapeutically. As a result, personalized medicine approaches have not yet been realized. However, clinical availability of numerous anti-myeloma drugs and readily obtainable bone marrow (BM) aspirates raises the possibility to benefit patients by profiling the drug sensitivity of their MM. Despite newly available drugs, resistance inevitably develops and there is no defined rationale for sequencing therapy. Treatments now consist of >15 agents, including IMiDs, protease inhibitors (PIs), alkylator chemotherapies, steroids, monoclonal antibodies, and options are still expanding. To profile sensitivity to the wide array of options, we developed a rapid functional assay of primary MM cells, distinct from prior assays by forgoing CD138+ cell selection to optimize MM cell viability and allow measurement of monoclonal antibody activity.
Methods: Myeloma drug sensitivity testing (My-DST) was performed on patient BM aspirates to measure the MM sensitivity in heterogeneous mononuclear cell (MNC) mixtures. My-DST is performed by culturing samples with a 7-drug panel consisting of bortezomib (Bor), carfilzomib (Car), lenalidomide (Len), pomalidomide (Pom), dexamethasone (Dex), cyclophosphamide (Cy, metabolite 4HC), and daratumumab (Dara) for 48h, followed by multiparameter, high-throughput flow cytometry to measure the surviving MM population. All conditions are performed in triplicate and in parallel, clonality verified by kappa/lambda staining.
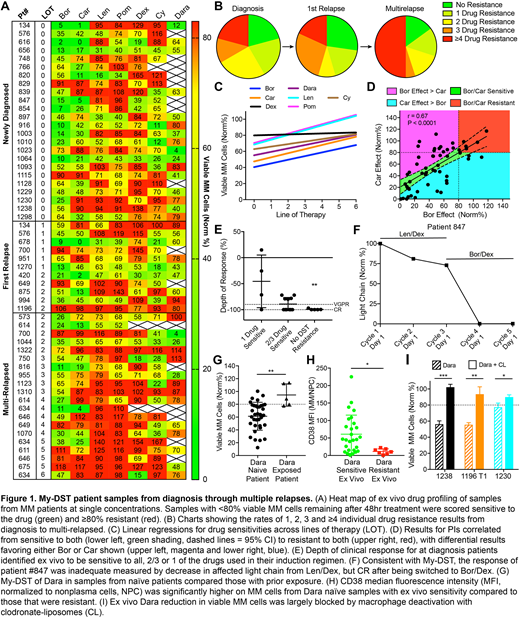
Results: MM cell viability was substantially better in unselected MNC cultures than CD138-selected cultures (data not shown). Dose-response was evaluated for each agent in MNC cultures to select single active concentrations for high throughput screening (data not shown). Single concentration profiling was completed on 55 patient samples from diagnosis (n = 24), first relapse (n = 11) and after multiple relapses (n = 20) with 2.5 nM PIs, 10 µM IMiDs, 1 µM Dex, 3.75 µM Cy and 20 nM Dara. Each was scored as sensitive or resistant using a cutoff of 80% surviving MM cells normalized to untreated controls (Fig 1A). Mild inherent resistance to varied tested agents was evident at diagnosis and progressively increased with lines of therapy (LOT) until multidrug resistance predominated (Fig 1B). My-DST displayed significant correlation for LOT with increasing resistance to Bor (r = 0.27, P = 0.046), Car (r = 0.27, P = 0.044), Len (r = 0.44, P = 0.0006) and Pom (r = 0.43, P = 0.0009), but not Dara (r = 0.27, P = 0.10), Cy (r = 0.17, P = 0.24) and Dex (r = 0.032, P = 0.82) (Fig 1C). The ex vivo sensitivity for Bor and Car were highly correlated (r = 0.67, P < 0.0001) from sensitive to both to resistant to both, but still differential results favoring Bor or Car were observed (Fig 1D). The ex vivo results for the tested IMiDs were even more highly correlated (r = 0.79, P < 0.0001), with fewer samples showing greater sensitivity differentially to Len or Pom (data not shown). In patients at diagnosis, the ex vivo sensitivity to all drugs given during treatment led to significantly deeper clinical responses than those with resistance to ≥1 drugs received (Fig 1E). As an example of potential clinical utility of My-DST, the test for #847 at diagnosis identified sensitivity to PIs and resistance to IMiDs, which correlated with inadequate response to Len/Dex, followed by complete response (CR) after change to Bor/Dex (Fig 1F). Dara had ex vivo activity in most naïve patients, but patients already exposed showed significant acquired resistance (Fig 1G). We also found that CD38 expression was significantly lower in patients that showed ex vivo Dara resistance (Fig 1H). The ex vivo Dara activity was partially blocked by inhibiting macrophages with clodronate-liposomes (CL) (Fig 1I).
Conclusion: My-DST on unselected MM BM aspirates with minimal perturbations ex vivo was clinically predictive of resistance to IMiDs and PIs. For the first time, this approach is able to measure the antibody-mediated cytotoxicity of daratumumab in clinical MM samples. Our data indicate that personalized regimens based on rapidly obtained ex vivo sensitivity may lead to optimized depth of clinical response. We next plan to translate this approach into clinical use in an effort to improve patient outcomes and avoid wasted time, money and complications that may arise from the use of ineffective drugs.
Mark:Janssen, Takeda, Celgene, Amgen: Consultancy; BMS, Celgene: Research Funding; Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal